Abstract
Introduction: The severe or moderately severe deficiency of coagulation factor IX (FIX) in patients with hemophilia B causes frequent and potentially serious bleeding, whereby joint bleeding can be one of the more serious complications. Management of bleeding episodes (BE) involves on-demand or prophylaxis replacement therapy for FIX. This study evaluated the safety profile and efficacy of a recombinant FIX, nonacog gamma (BAX326, RIXUBIS®, Shire, Lexington, MA, USA), for treatment of bleeding in adults and children with severe (FIX level <1 IU/dL) or moderately severe (FIX level 1-2 IU/dL) hemophilia B.
Methods: This phase 3, prospective, open-label, multicenter, continuation study (NCT01286779) comprised patients from a phase 1/3 pivotal study (NCT01174446), a phase 2/3 pediatric study (NCT01488994), and naïve patients aged 2-70 years at screening. Nonacog gamma treatment was administered at the discretion of the investigator. Patients who transferred from earlier studies received on-demand treatment or prophylaxis, while naïve patients received only prophylaxis. Prophylactic dosing was standard (SP; 40-60 IU/kg in patients ≥12 years of age or 40-80 IU/kg in patients <12 years of age, twice weekly), modified (MP; ≤100 IU/kg, as determined by the investigator), or tailored to the patient's pharmacokinetic profile (PK; ≤120 IU/kg). One dose (75 ± 5 IU/kg) was given at each of the study visits to assess incremental recovery of nonacog gamma over time. The primary outcome measure was the occurrence of adverse events (AE) and serious AEs (SAE), regarded as possibly or probably related to nonacog gamma. Secondary outcomes included additional safety, annualized bleeding rate (ABR), hemostatic efficacy rating at resolution of bleed, and number of infusions per bleeding episode.
Results: The study enrolled 117 patients, from 40 sites in 16 countries, of which 115 patients (74 patients with severe hemophilia, and 41 patients with moderately severe hemophilia) received treatment with nonacog gamma. All patients were male, with 21 patients <12 years of age and 94 patients ≥12 years of age.These included 65 patients from the phase 1/3 pivotal study, 20 patients from the phase 2/3 pediatric study and 30 naïve patients.
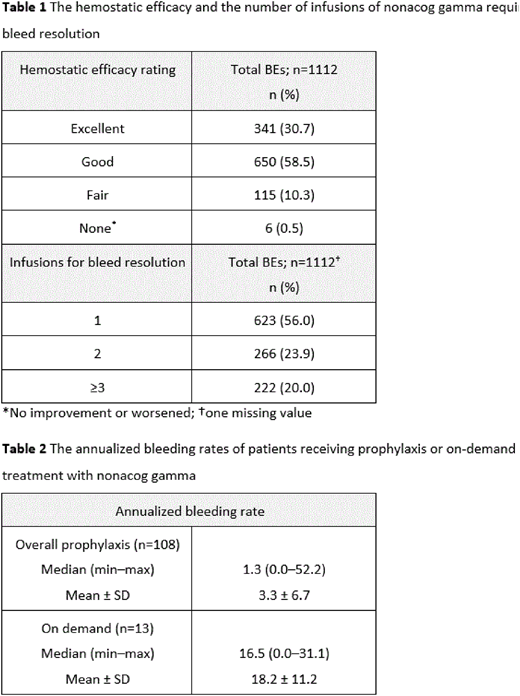
Of 115 patients included in the full ananlysis set, 110 were treated with a prophylaxis regimen (SP, 108 patients; MP, 26 patients; PK, 3 patients ) and 13 patients received nonacog gamma on demand. Patients could switch between treatment regimens and may therefore be included in more than one regimen. A total of 459 AEs were reported in 85 (73.9%) patients; 443 nonserious AEs in 85 patients and 16 SAEs in 9 patients. The most commonly reported AEs included: nasopharyngitis (55 in 25 patients), arthralgia (48 in 15 patients), and pyrexia (23 in 14 patients). Only 2 nonserious AEs (positive antibody (Ab) tests to rFurin) in 2 patients, were considered nonacog gamma-related. These tests were negative by the time of study completion and considered transient. None of the SAEs were deemed related to nonacog gamma. No patients developed Abs to FIX, there were no thrombotic events or severe allergic reactions during or after treatment, and no significant treatment-related changes in vital signs. Clinical efficacy of nonacog gamma was rated as excellent or good in 991 (89%) of 1112 BEs. For all BEs, a mean (± SD) number of 1.8 (± 1.65) infusions were required until bleed resolution (Table 1). For on-demand treatment, the median ABR was 16.5 (n=13), whereas for overall prophylaxis the median ABR was 1.3 (n=108) (Table 2).
Windyga:Alnylam, Baxalta, Bayer, Novo Nordisk, Octapharma, Rigel Pharmaceuticals, Roche, Sanofi, Shire, SOBI: Research Funding; Alexion, Baxalta, Bayer, CSL Behring, Ferring Pharmaceuticals, Novo Nordisk, Octapharma, Rigel Pharmaceuticals, Roche, Sanofi, Shire, Siemens, SOBI, Werfen: Membership on an entity's Board of Directors or advisory committees. Stasyshyn:CSL Behring, Novonordisk, Shire: Honoraria; Novonordisk, Pfiser , Shire: Speakers Bureau. Engl:Shire: Employment, Equity Ownership. Tangada:Shire: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.